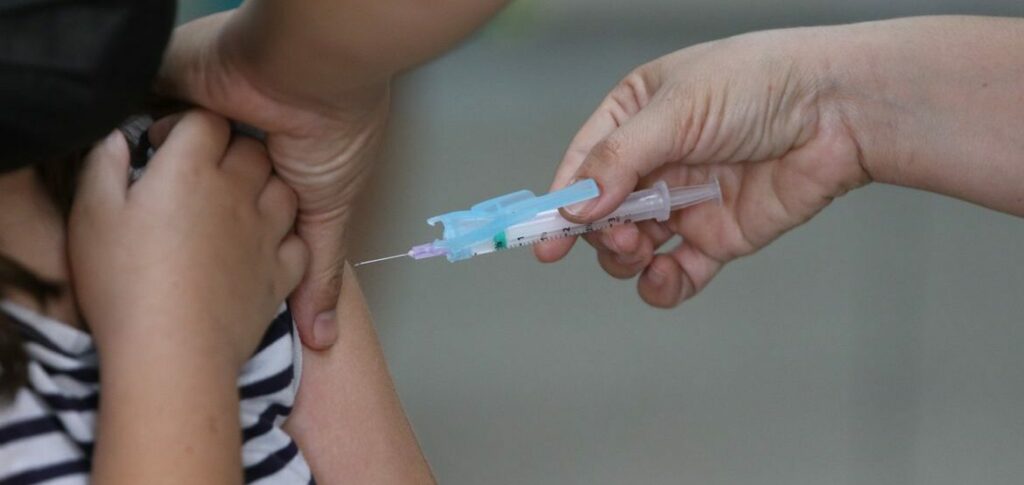
According to Anvisa, the new vaccine is made up of four different serotypes of the virus that causes the disease, which guarantees broad protection against it. Last year, Brazil recorded more than a thousand deaths from dengue complications in the country.
ADVERTISING
Last month, the National Technical Commission on Biosafety (CTNBio) approved the safety of the Qdenga vaccine, which was now awaiting approval from Anvisa.
Another vaccine against dengue already approved in the country, Dengvaxia, can only be used by those who have already had the disease.
The Qdenga vaccine was also evaluated by the European health agency (EMA), from which it also received approval. The granting of registration by Anvisa allows the product to be sold in the country, as long as the approved conditions are maintained. The vaccine, however, will continue to be subject to monitoring of adverse events through pharmacovigilance actions under the responsibility of the company itself.
ADVERTISING
(With Brazil Agency)