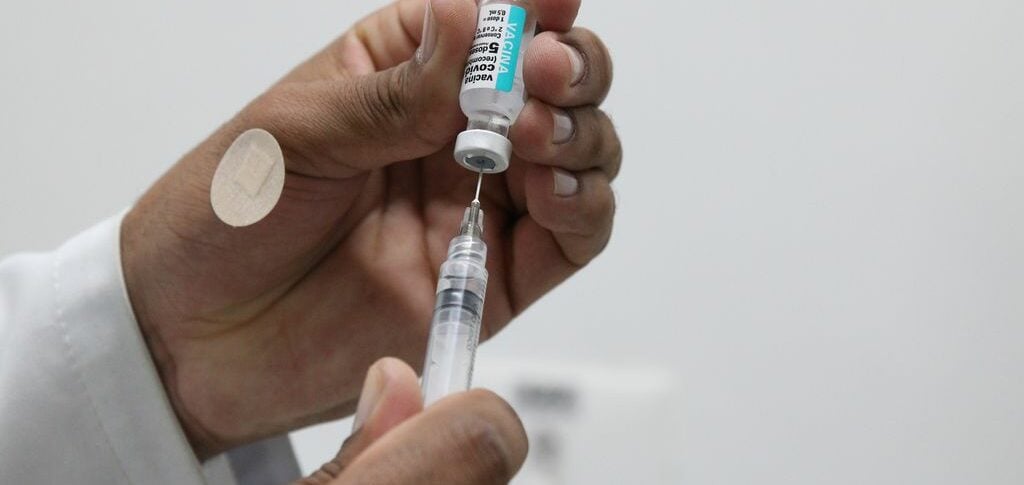
During the meeting, the bodies analyzed information about an updated version of Coronavac, manufactured by Chinese biopharmaceutical company Sinovac.
ADVERTISING
According to Butantan, to date, data on this production are still preliminary, as Sinovac has only conducted two pre-clinical studies. The tests were only carried out on animals, but Sinovac is already recruiting volunteers to continue with the human testing stage.
Anvisa took the opportunity to signal which points should be observed in a future authorization request for the trivalent vaccine against Covid-19.
The Butantan Institute left its commitment to formalize, with Anvisa, the data that is currently available on the new vaccine and also the planning proposed by Sinovac regarding the next phases of clinical studies. Then, the regulatory agency must analyze the proposals.
ADVERTISING
Source: Estadão Content
See also: